RELIABLE PARTNER FOR CONTRACT MANUFACTURING
JEmedis as a CDMO
At JEmedis, we are here to support you with our wide-ranging expertise. We are also, and foremost, a trustworthy partner in the area of CDMO. As a contract manufacturer, we offer the support you need: from process development to the finished product in the R&D area and pilot scale.
CDMO
A Contract Development and Manufacturing Organisation (CDMO) is a company that provides comprehensive services from the development to the manufacture of pharmaceuticals on behalf of other companies. Pharmaceutical and biotechnology companies use CDMOs to reduce fixed costs, simplify supply chains and shorten R&D lead times.
JEmedis is the Ideal Partner
This is where we come into play as an innovative new stakeholder. JEmedis is perfectly equipped to steer you through the early stages of process development and produce pilot-scale batches. We bring our expertise in innovative product development to our customers’ projects and offer them tailor-made processes. In this way, we support you in optimising and accelerating manufacturing steps and reducing risks.
We look forward to the challenges of the future and are always available to provide our customers with advice and support.
Biologics:
Process Development, Contract Manufacturing and Analytics
You would like to have your product manufactured on an R&D laboratory scale or to redesign and optimise your manufacturing process?
Then you have come to the right place. We support our customers in the development, implementation, and optimisation of their manufacturing processes with the aim of producing a safe product with consistent quality. We have specialised in single-use systems.
You have an existing manufacturing process and would like to have your drug produced on a pilot scale for testing purposes or for preclinical or clinical studies?
Then we are the right partner for you. In our modern GMP clean room facility, we offer our customers an aseptic fill & finish service for their product in small batches upon request, including approval from our in-house expert.
Our Services at a Glance:
In our modern cleanroom facility (RK D to B(A)) we offer support in the development and optimisation of manufacturing processes for biologics based on an existing manufacturing design as well as contract manufacturing of pilot batches (cGMP and non-GMP), including:
Upstream & Downstream
- Microbiological cultivation and fermentation of bacteria including risk group 2
- Production in state-of-the-art clean rooms
- Development of transfer lines for aseptic transfer
- Filtration & Formulation
- Scaling up from laboratory scale to pilot scale
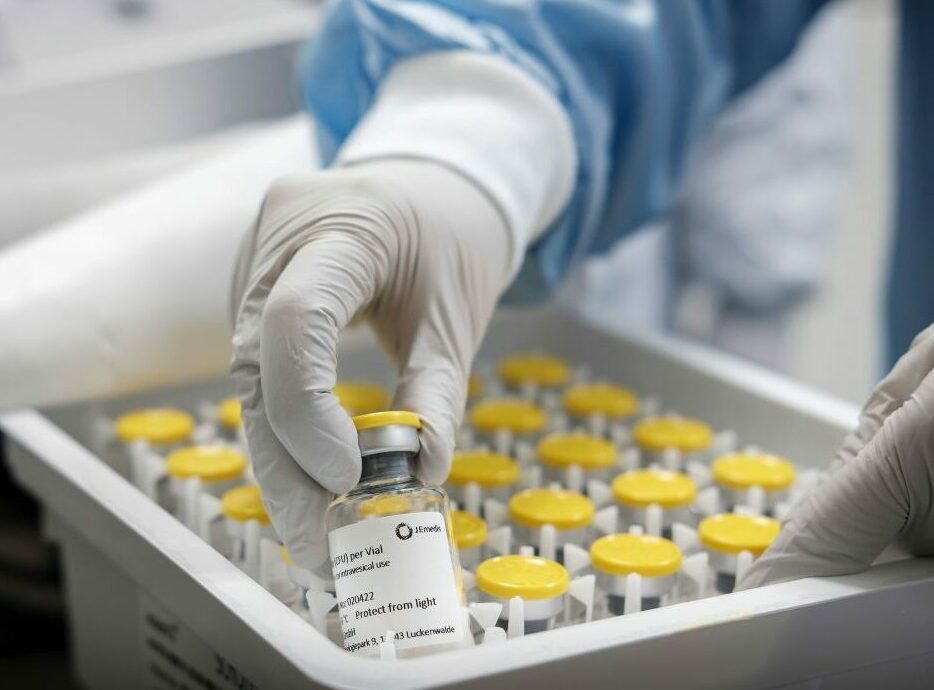
Aseptic Fill & Finish
- Semi-automatic aseptic filling from 0.5 to 50 ml filling volume using different vial sizes
- Freeze-drying integrated in the A area with a 1.05 m² footprint and a capacity of 4000 2 R vials or 1000 20 R vials per batch
- Semi-automatic crimping of 13 mm and 20 mm closures
- Labelling of vials (for investigational medicinal products)
Analytics
We work with our reliable and GMP-certified partner laboratory for quality control and analytical testing of the products/medicines. Tests can be carried out in the areas of microbiology, analysis, and molecular biology, which are approved by the head of our quality management process.
Support & Advice
Based on our experience in this area and in close cooperation with our suppliers and service managers, we can support you in choosing the suitable SU-Equipment, primary packaging, and with other relevant topics.